Trends & Reports
Trends & Reports
Batteries have evolved from simple energy storage devices into a driving force for sustainability, efficiency, and technological advancement. But the journey does not stop here. The demand for faster charging, longer-lasting, lighter, and safer batteries is continuously growing. Next-generation batteries will play a pivotal role in turning imagination into reality. The mobility revolution, which began with electric vehicles, is now expanding into urban air mobility (UAM), pushing for faster and safer modes of transportation. This shift represents not just technological progress, but a fundamental change in how we live, commute, and travel.
| Overcoming liquid electrolyte limits: The promise of all-solid-state batteries
While lithium-ion batteries have been widely successful and offer numerous advantages, they do face some limitations, particularly with their “liquid electrolytes.” These electrolytes are susceptible to temperature fluctuations, which can lead to volume changes and pose a leakage risk when subjected to external shocks. These challenges directly impact battery safety, driving the industry to explore new alternatives.
This is where all-solid-state batteries come into play. As the name suggests, these batteries use solid materials for all components. This technology enhances resistance to temperature variations and external shocks, significantly improving safety and durability. Additionally, all-solid-state batteries offer higher energy density, enabling longer mileage for EVs and paving the way for next-generation energy solutions.
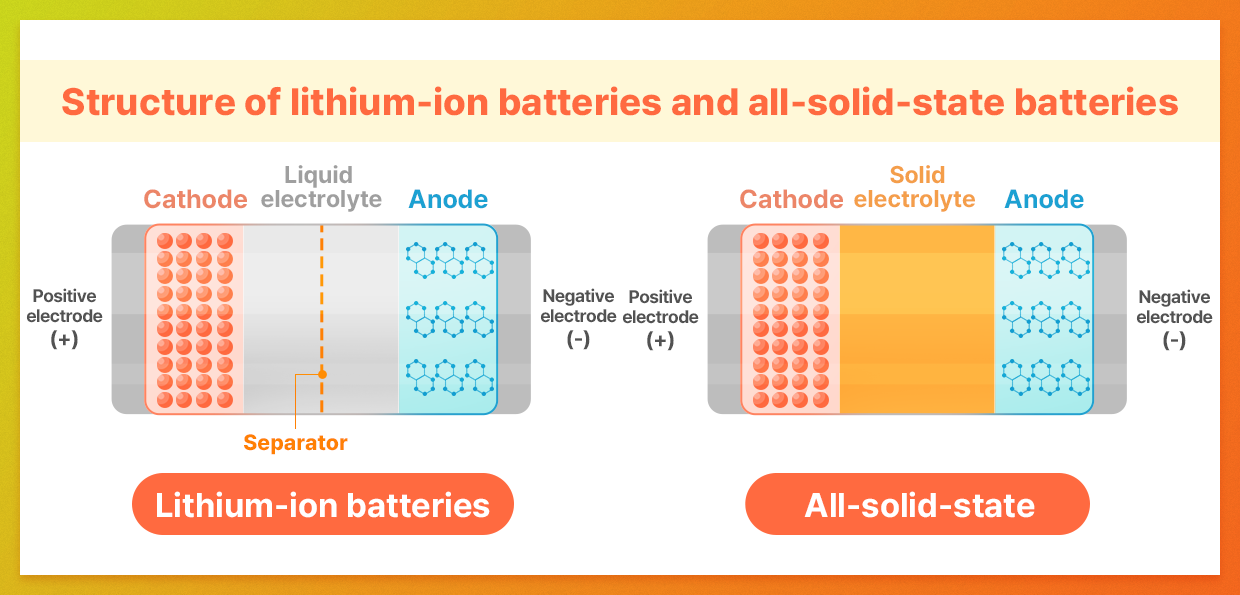
All-solid-state batteries offer four main advantages. First and foremost is safety. By using solid electrolytes, these batteries fundamentally eliminate the risks of fire and explosion associated with electrolyte leakage.
The second advantage is high energy density. Unlike conventional batteries that require a separator to keep the anode and cathode apart, all-solid-state batteries use the solid electrolyte itself as the separator. This design conserves space, enabling more active materials to be packed into the same-sized battery, which can significantly extend the driving range.
The third advantage is a wide operational temperature range. Lithium-ion batteries experience reduced ionic conductivity(1) below -10°C, leading to diminished performance and an increased risk of thermal runaway at higher temperatures. In contrast, all-solid-state batteries maintain stability between -40°C and 100°C, significantly broadening their potential applications.
(1) Ionic conductivity: a material’s ability to conduct ions, with higher values indicating easier ion movement within the electrolyte.
Lastly, all-solid-state batteries offer high power output. These batteries enable the use of a bipolar electrode structure and allow multiple electrodes to be stacked within a single cell. This design increases voltage and enhances power output, making all-solid-state batteries ideal not only for automobiles but also for high-energy-demanding applications such as industrial machinery, trains, aircraft, and even space exploration equipment.
However, realizing these advantages comes with its own set of challenges. The battery industry faces critical technical hurdles, such as the slower ion transport speed in solid electrolytes and the dendrite(2) phenomenon. To overcome these barriers, extensive research is being conducted on various types of solid electrolytes. Polymer-based, oxide-based, and sulfide-based materials are at the forefront of this development.
(2) Dendrites: branch-like crystalline structures that form on the anode surface during charge/discharge cycles as lithium ions travel between the cathode and anode. These structures are a major cause of reduced battery lifespan and safety issues.
Polymer-based electrolytes have characteristics similar to conventional liquid electrolytes, which makes them easy to integrate into existing process designs and cost-effective. They can be processed into various forms, offering flexibility in manufacturing. However, due to their low ionic conductivity, they have limitations in power output as EV batteries.
Oxide-based electrolytes offer relatively high ionic conductivity and strong chemical stability with various materials. However, they require processing temperatures exceeding 1,000°C, which poses significant challenges for commercialization. To address this issue, efforts are being made to develop hybrid electrolytes that combine polymer-based and oxide-based systems.
Sulfide-based electrolytes provide high ionic conductivity and energy density, making them some of the most extensively studied materials in solid-state battery research. However, there are concerns regarding their safety, such as the potential release of toxic hydrogen sulfide (H₂S) gas when exposed to moisture and incompatibility with existing manufacturing processes. Consequently, active research and development are underway to address these challenges and ensure safe and efficient integration into battery systems.
☝️ The Pursuit of innovation in solid electrolyte materials
Despite technical barriers facing all-solid-state batteries, SK On is making strides to overcome these challenges through continuous research and global partnerships. The company is currently developing two types of solid-state batteries: polymer-oxide hybrids and sulfide-based batteries. With a focus on capturing the next-generation battery market, SK On aims to produce pilot prototypes by 2025 and 2026, respectively, with commercial prototypes planned by 2028 and 2029.

▲ Solid-state battery prototype unveiled by SK On at InterBattery 2024, Coex, Seoul, March 2024
In August 2023, SK On partnered with a team under Professor Park Hee Jung from the Department of Materials Science and Engineering at Dankook University to successfully develop a novel oxide-based solid electrolyte with the world’s highest level of lithium-ion conductivity. This electrolyte not only enhances lithium-ion conductivity, but also ensures stability in atmospheric conditions By employing technology that uniformly controls the microstructure of LLZO(3), SK On has resolved stability issues related to increased lithium-ion conductivity and addressed vulnerabilities of existing solid electrolytes to moisture (H2O) and carbon dioxide (CO2).
(3) LLZO (Li-La-Zr-O): Lithium-Lanthanum-Zirconium-Oxygen
SK On is actively strengthening its global collaborations in the development of sulfide-based electrolytes. In January 2024, the company signed a technology transfer agreement with Solid Power, U.S.-based solid-state battery specialist, to accelerate the advanced development of solid-state batteries. This partnership focuses on enhancing key performance metrics, such as lifespan and energy density.
✌ Innovation in anode materials: From graphite to lithium Metal
Currently, most batteries use graphite as an anode material. lithium metal batteries replace conventional liquid electrolytes with solid electrolytes and use lithium metal as an anode material instead of graphite. This breakthrough technology is expected to significantly increase energy density and maximize lithium-ion transport efficiency, leading to substantial improvements in the driving range and the charging speed of electric vehicles.
However, there are technical challenges that need to be addressed before the commercialization of lithium metal batteries. Pure lithium metal tends to overreact with electrolytes and may form irregular dendrites on its surface during charging, which compromises both performance and safety. To tackle these issues, SK On has developed a polymer electrolyte known as SIPE (Single-ion Conducting Polymer Electrolyte).
SIPE offers approximately 10 times higher ionic conductivity at room temperature and a lithium-ion transference number(4) about five times greater than conventional polymer electrolytes. By forming a stable SEI layer(5), it ensures a reliable interface between the lithium metal and the polymer electrolyte, effectively minimizing dendrite formation. Furthermore, SIPE delivers stable performance at room temperature and retains 77% of its initial capacity even during rapid charge/discharge cycles. With superb mechanical durability, SIPE is suitable for mass production and remains stable at temperatures exceeding 250°C, making it an electrolyte with broad application potential.
(4) Transference number: the proportion of charge carried by ions during conduction. Higher lithium-ion transference numbers indicate an increase in lithium-ion movements.
(5) SEI (Solid Electrolyte Interphase): a thin layer formed on the anode surface during the initial battery charge.
| Power from salt? The rise of sodium-ion batteries
Sodium-ion batteries are gaining attention due to their use of sodium, a resource 500 times more abundant on Earth than lithium. Unlike lithium, which is limited and concentrated in a few countries, sodium is plentiful and can be extracted from seawater almost anywhere in the world.
So, why haven’t sodium-ion batteries been widely commercialized yet? One reason is that sodium atoms are larger and heavier than lithium atoms, leading to lower energy density and increased weight for batteries of the same size. To overcome these limitations, a new approach, which aims to combine sodium with lithium iron phosphate (LFP) and integrate two types of batteries into a single pack, is under development. This structure leverages the strengths of both materials to offset their individual weaknesses.
Despite these advancements, sodium-ion batteries currently achieve only 40-50% of the energy density of widely used lithium-ion batteries. As a result, they are not well-suited for high-energy-demand applications, particularly in electric vehicles, where energy density is a crucial factor.
| Cobalt-free batteries: A step toward sustainability
Cobalt is crucial in preventing cathode corrosion and controlling explosion risks in batteries. However, its supply has become increasingly unstable, with prices skyrocketing in recent years. In terms of accessibility, approximately 80% of the world’s cobalt reserves are concentrated in the Democratic Republic of Congo, posing significant risks to supply chain stability.
In response, the battery industry is developing cobalt-free (Co-Free) batteries by increasing the proportion of manganese (Mn) or nickel (Ni) in cathode materials for ternary batteries. Among these, High-Manganese (High-Mn) or Manganese-Rich (Mn-Rich) batteries, which significantly boost the lithium and manganese content, respectively, are emerging as leading cobalt-free technologies
Similar to cobalt, manganese enhances stability of a battery and reduces explosion risks, while offering a significant cost advantage. At only about 5% of cobalt’s price, manganese can considerably reduce battery production costs. Since batteries account for roughly 40% of an electric vehicle’s price, High-Manganese batteries could play a pivotal role in making EVs more affordable, thereby driving widespread EV adoption. Additionally, these batteries maintain adequate energy density, positioning them as promising contenders for game-changing innovations in the low-cost EV market.
SK On’s cobalt-free battery technology received the Bronze Award in the “Smart Transportation” category at the 2024 Edison Awards, highlighting its technological excellence. By addressing the challenges of structural instability and lifespan degradation in cobalt-free batteries using single-crystal cathodes and proprietary doping technology, SK On received praise. Furthermore, at the 2023 InterBattery event, the company unveiled its cobalt-free battery prototype, capturing the industry’s attention.

▲ Cobalt-free battery unveiled by SK On at InterBattery 2023, Coex, Seoul, March 2023
☝ Lithium-sulfur batteries: In the spotlight of market interest
Lithium-sulfur batteries are emerging as a highly promising next-generation technology alongside lithium-metal batteries for potential commercialization. These batteries use sulfur as cathode material and lithium metal as the anode. Sulfur, the 17th most abundant element on Earth, offers an alternative to expensive cobalt, significantly reducing manufacturing costs. Additionally, both sulfur and lithium metal have low densities and high capacity per unit weight, providing energy density up to five times greater than that of conventional lithium-ion batteries theoretically.
However, like other emerging technologies, lithium-sulfur batteries also have challenges. The combination of lithium and sulfur generates lithium polysulfides, which can dissolve into the electrolyte and lead to performance issues as well as battery lifespan degradation.
✌ Lithium-air batteries: The possibility of air-powered energy
Environmental battery technologies are gaining momentum, and lithium-air batteries are emerging as revolutionary innovations. As the name suggests, these batteries use oxygen from the air as cathode material and lithium metal as the anode. This unique design makes them lighter and more compact than any other batteries. Notably, they feature the highest energy density among currently known technologies, potentially leading to breakthroughs in next-generation batteries. However, their distinctive structure—which includes gaseous cathodes, liquid electrolytes, and solid anodes—poses significant challenges. Therefore, additional research is essential to optimize their charge and discharge efficiency.
Technological progress often goes beyond our imagination—Can the development of the next-generation batteries follow a similar path? The race for faster, safer, and longer-lasting batteries is well underway!










 Youtube
Youtube Facebook
Facebook Instagram
Instagram Linkedin
Linkedin