2025.01.17
 Trends & Reports
Trends & Reports
In October 2019, the world witnessed the oldest Nobel Prize winner ever. This remarkable person was John B. Goodenough, professor at the University of Texas at Austin, who won the prize at the age of 97, and passed away in 2023. He was jointly awarded the Nobel Prize in Chemistry alongside with M. Stanley Whittingham, professor at Binghamton University, State University of New York, and Akira Yoshino, honorary fellow at Meijo University in Japan.
These three scientists were recognized by the Nobel Committee for their great contribution in the development of lithium-ion (li-ion) batteries, a type of rechargeable/secondary batteries. The history of secondary batteries dates back to the 1850s, but their widespread use began only after the development of li-ion batteries. Their importance was highlighted in the official press release of The Nobel Prize in Chemistry 2019 as follows:
“This lightweight, rechargeable and powerful battery is now used in everything from mobile phones to laptops and electric vehicles. It can also store significant amounts of energy from solar and wind power, making possible a fossil fuel-free society.”
| The 4 key components of secondary batteries
Secondary batteries exhibit remarkable properties in the course of two processes: discharging and charging. Discharge takes place when oxidation occurs at the anode (negative electrode), and reduction occurs at the cathode (positive electrode). It makes electrons move from the anode to the cathode. Conversely, during charging, electrons move from the cathode to the anode, powered by an external source, and pushing chemical reactions in the opposite direction. In the case of Lithium-ion batteries, battery charges when lithium ions move to the anode, and discharges when lithium ions return to the cathode.
The four key components of secondary batteries are the cathode, anode, separator, and electrolyte.
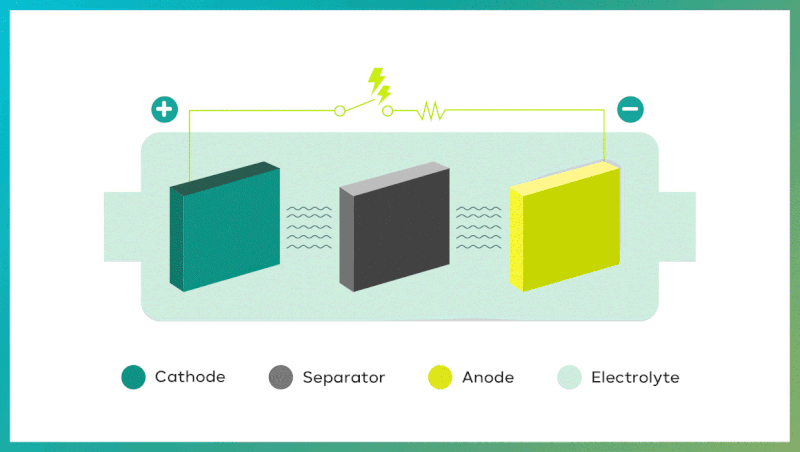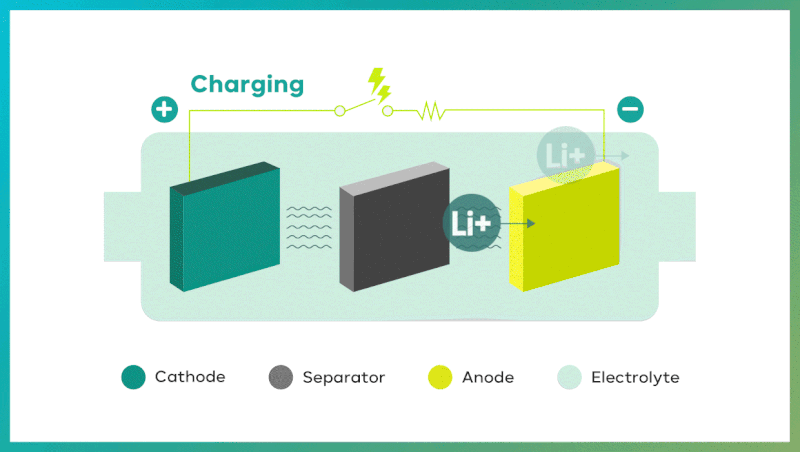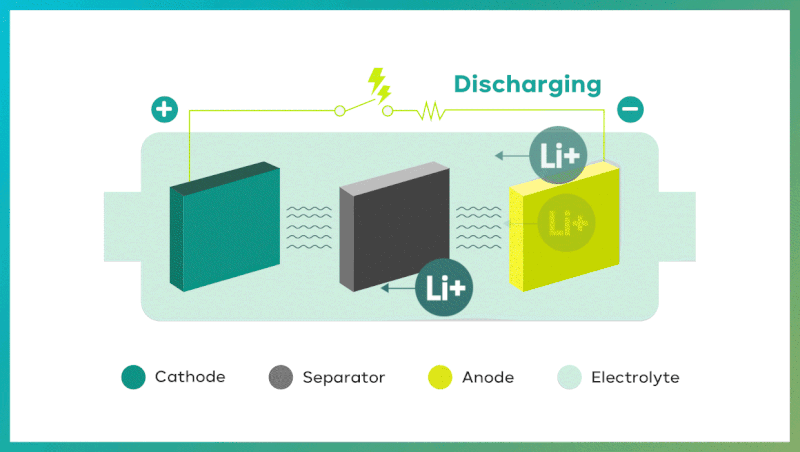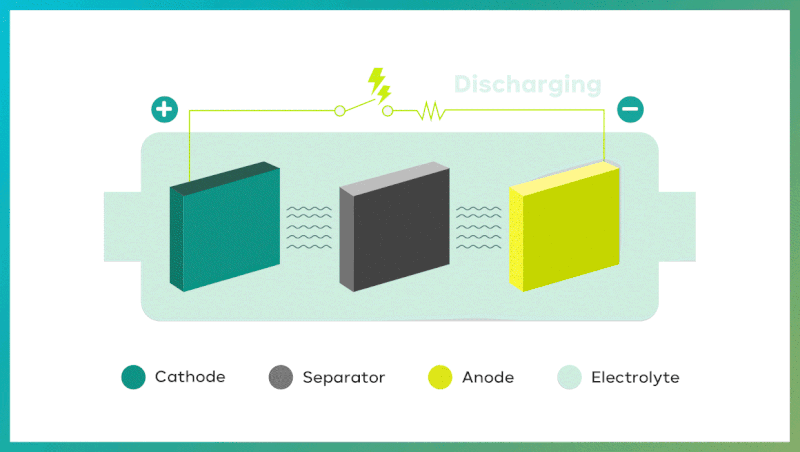
| Cathode: determining li-ion battery capacity and output
Cathode is the positive (+) electrode where the essential lithium for li-ion batteries resides, and during charging, lithium ions lose electrons, and oxidize at this electrode. On the contrary, during discharging, lithium ions gain electrons and reduce at this electrode. The cathode is made up of several components which are a current collector* (aluminum foil), cathode active material, conductive additive**, and binder. The cathode active material includes various metal elements such as lithium, nickel (Ni), cobalt (Co), manganese (Mn), iron (Fe), and aluminum (Al). The combination of these elements determines battery’s capacity and output.
*Current collector: thin film about 10μm thick inside the battery with low electrical resistance, designed to conduct current to and from the active material during charging and discharging.
**Conductive additive: substance that accelerates electron movement between the anode active material and the cathode active material.
Also, based on the combination of the mentioned above active materials, battery types are classified as follows:
● LCO (Lithium Cobalt Oxide) battery
● LMO (Lithium Manganese Oxide) battery
● LFP (Lithium Iron Phosphate) battery
● NCM (Nickel Cobalt Manganese) battery
● NCA (Nickel Cobalt Aluminum) battery
● LTO (Lithium Titanate Oxide) battery
| Anode: determining lifespan and charging speed of li-ion batteries
Anode is the negative (-) electrode where lithium ions are reduced by gaining electrons during charging, and are oxidized by losing electrons during discharging. This is the opposite of the process that occurs at the cathode. The anode electrode consists of a current collector, aluminum foil, anode active material, conductive additive, and binder.
Among these, anode material plays a major role in determining lifespan and charging speed. The more lithium ions anode material can store, the longer battery lifespan. Moreover, the better it acquires lithium ions, the shorter charging time. The overall performance of a cell actually largely depends on the performance of the anode material battery.
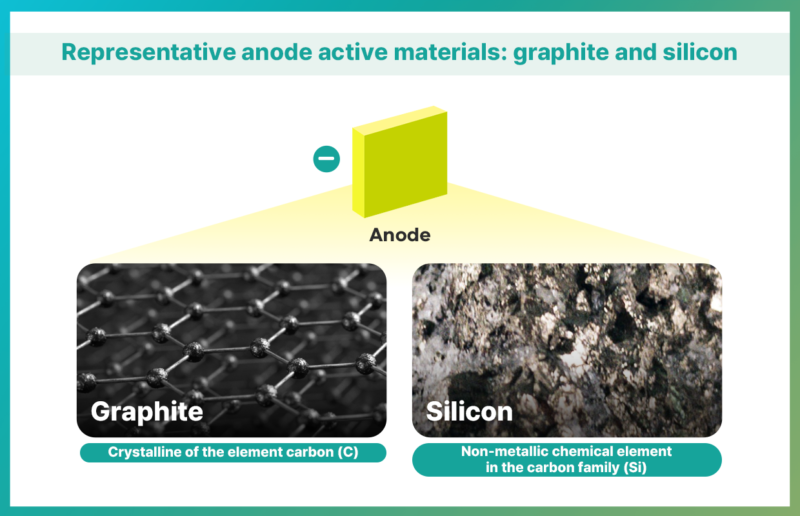
Currently, the most commonly used material for anode active material is graphite due to its affordable cost and stability. The graphite used in anodes is broadly divided into natural and artificial graphite. Natural graphite is inexpensive to procure since it can be sourced from nature and generally has a larger capacity. However, during the charging process of li-ion batteries, lithium ions moving from the cathode to the anode material stay between graphite layers, causing graphite to expand and potentially changing the battery’s structure. To improve it, synthetic graphite has been developed. Synthetic graphite offers superior charging speed and output compared to natural graphite, but it is more expensive to produce and has a slightly lower capacity.
Recently, silicon has emerged as a next-generation anode material. It is because silicon anode materials have about 10 times more powerful energy density of graphite-based anode materials, which not only increases battery capacity, but also reduces charging time. While carbon found in graphite can store 1 lithium ion per 6 carbon atoms, silicon can combine with lithium ions and store a total of 22 lithium ions per 5 silicon atoms. Therefore, as the proportion of silicon increases, both battery capacity and charging speed improve, making silicon anode material highly effective.
| Separator: ensuring safety inside li-ion cells
Separator is a microporous film placed between the anode and cathode to stop short-circuiting within the battery while charging and discharging. It prevents internal short circuits during charging and discharging, ensuring that only ions pass from one electrode to the other, preventing electrons from moving in the wrong direction. It consists of a base film that plays a significant role in safety and performance, and a coating layer which guarantees thermal stability of separator.
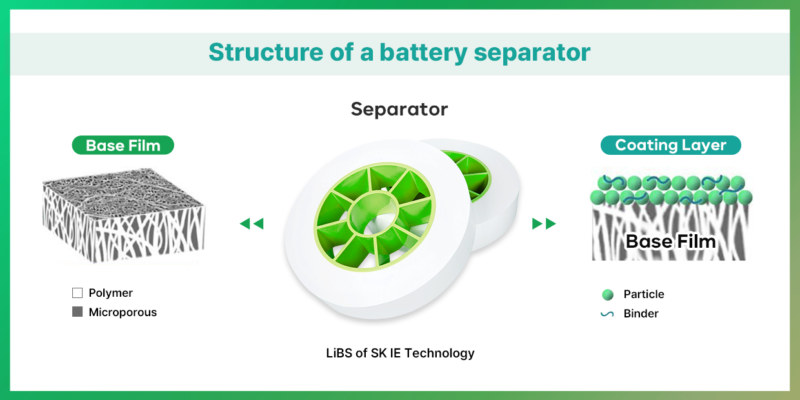
Depending on process principles, separators are generally divided into the Wet and the Dry process. Wet-laid separators are made using Thermally Induced Phase Separation (TIPS)*** method involves mixing oil with Polyethylene (PE) and Polypropylene (PP), kneading them at high temperature and pressure, and then cooling down, allowing oil components to separate. The oil components are afterward removed to create pores. Wet-laid separators can produce separators with high energy density, high capacity, high performance, and thin membranes with uniform pore size and distribution. However, they are less advantageous due to their high-cost production.
***Thermally Induced Phase Separation (TIPS): a manufacturing method using a solvent that dissolves at high temperature. The separator is created through temperature differences, and the pore size is determined by the type of solvent applied.
Dry-laid separators are made by kneading PE and PP at high temperature and pressure, then cooling them down to form crystals. These crystals are mechanically stretched to create pores through a relatively simple manufacturing process. They have excellent conductivity and resistance to high temperature as well as oxidation. However, they have some disadvantages such as uneven pore size or lower stability.
| Electrolytes: transporting ions in li-ion cells
The electrolyte in li-ion batteries fills the space between the cathode and anode, acting as a medium for moving lithium ions. During battery charging and discharging, lithium ions migrate through the electrolyte between the cathode and anode. To transport ions in a quick and safe way, the electrolyte must have excellent chemical and electrical stability, low freezing point, and high flash point to work effectively over a wide range of temperatures. Additionally, the so-called “ionic conductivity,” which refers to the speed of ion movement within the electrolyte, should be high because faster lithium ion movement improves battery performance and speeds up charging.
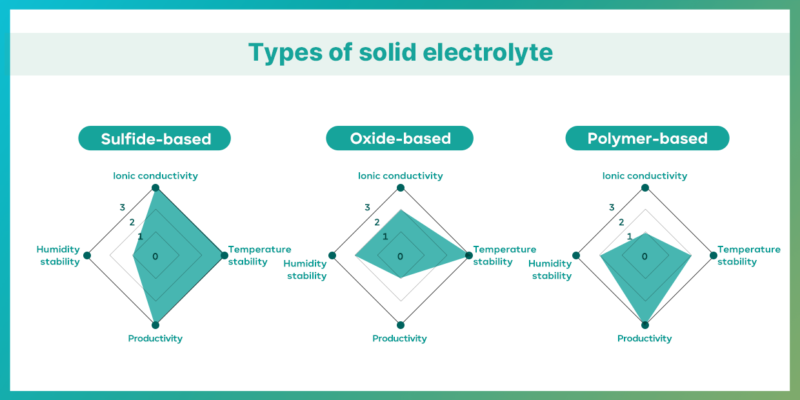
Electrolytes are generally divided into liquid and solid types. In li-ion batteries, liquid electrolytes consist of lithium salts (pathways for lithium ions), organic solvents (dissolve the lithium salts), and additives (determine the electrolyte’s characteristics).
The all-solid-state battery, which gains attention as the next-generation battery, uses solid electrolytes instead of liquid ones. As a result, it does not require a separator to prevent contact between the cathode and anode, providing higher safety and energy density compared to conventional secondary batteries such as li-ion batteries. Solid electrolytes are classified into sulfide-based, oxide-based, and polymer-based types based on the raw materials composition.
So far, we have examined the four key components of secondary batteries which are cathode, anode, separator, and electrolyte. In brief, cathode determines battery density, anode influences capacity and performance, separator ensures safety, and electrolyte acts as a mediator for electron movement. A superior secondary battery can be produced only when these four key components work together in synergy.
| “Battery Explorer” with SK Innovation affiliates’ products and technologies
SK On, the battery business subsidiary of SK Innovation, has been recognized for the highest level of technology in the NCM battery field. It leads the market by developing high-nickel batteries, including NCM622 (60% nickel, 20% cobalt, 20% manganese), NCM811 (80% nickel, 10% cobalt, 10% manganese), and the highest specification for mid-to-large li-ion batteries, NCM9+ (90% nickel, 5% cobalt, 5% manganese), which were developed first in the world. Furthermore, SK On is working on two types of all-solid-state batteries, polymer-oxide composite and sulfide-based, which are expected to be pilot produced by 2025 and 2026, and commercial prototypes by 2028 and 2029 respectively. The sulfide-based next-generation battery pilot plant, currently under construction at SK On’s Battery Research Institute in Daejeon, South Korea, is scheduled to be completed in the second half of 2025.
SK IE Technology, the materials business subsidiary of SK Innovation, is a leader in the global wet-laid separator market with its cutting-edge technology. The company is the first in the world that successfully developed Sequential Stretching Technology, which allows separators to be stretched uniformly in both longitudinal (front-back) and transverse (left-right) directions, resulting in separators with precise properties and thickness. Furthermore, SK IE Technology’s self-developed Ceramic Coated Separator (CCS) technology remarkably enhances durability of the separator. By coating the base film of the separator with ceramic particles, CCS prevents deformation and shrinkage under the heat generated by large-capacity batteries, thereby significantly minimizing fire risk.
Secondary batteries have long powered our portable devices like laptops or smartphones, becoming indispensable in our daily lives. Now, their applications are expanding into various industrial fields, unlocking new and exciting possibilities. Stay “charged” for more insights in the next article of Battery Explorer series!










 Youtube
Youtube Facebook
Facebook Instagram
Instagram Linkedin
Linkedin