Trends & Reports
Trends & Reports
In the early 1900s, French artists depicted visions of the year 2000 in a series of illustrations titled 「En L’An 2000」, imagining future innovations such as 「Electric Train」 and 「Auto Rollers」. Similarly, in 1965, about 9,000 kilometers away from France, a science fiction cartoonist named Lee Jung-moon introduced electric cars in his comic “Life in the Year 2000,” which was published in a science magazine in South Korea. These artists and visionaries from the East and West both dreamt of future transportation using electricity, illustrating how universal the fascination with electric-powered mobility was across cultures.

(Left) In the early 1900s, French artists depicted visions of the year 2000 in a series of illustrations titled “En L’An 2000”: (Top left) An illustration of future “Electric train from Paris to Beijing” (“Le train électrique Paris-Pékin”), released in the late 1890s to early 1900s; (Bottom left) An illustration of future “Auto Rollers” (“Auto-patins à roue”), released around 1910 (Source: Bibliothèque nationale de France) / (Right) In 1965, Korean cartoonist Lee Jung-moon explored similar themes in his comic “Life in the Year 2000,” published in a science magazine (Source: 2017, Press Release by the Ministry of Future Creation and Science)
| Once a Dream, Now a Reality
Nowadays, the various forms of electric transportation once envisioned by past artists have become an integral part of our daily lives. The key to realizing these dreams has been the technological development of “cell”, also known as a “battery”. From the view of a Battery Explorer, we aim to inspect not only the technological advancements in the revolution of batteries but also the impacts of these developments on our lives.
| Types and Characteristics of Batteries
We are already familiar with several types of battery, as our daily life is now surrounded by many electrical devices. From digital clocks, laptops, cell phones to cars, they all run with batterries but those cells are not quite the same. Based on their fundamental operating principles, they are broadly classified into chemical, biological, and physical batteries.
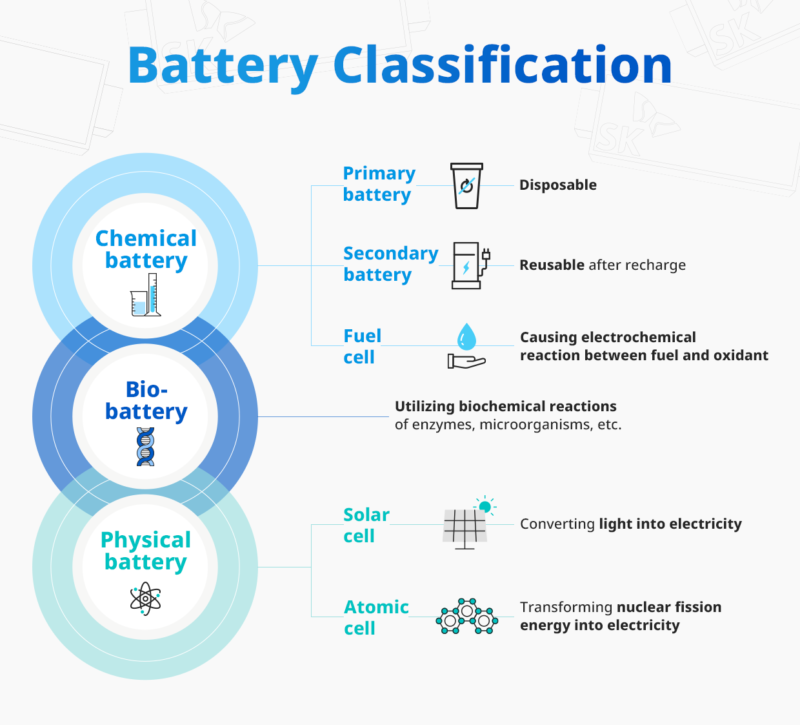
A chemical battery converts the energy generated from a chemical reaction into electrical energy. The materials involved in a chemical reaction are all present within the battery itself and can be further classified into primary battery, secondary battery, and fuel cell.
Primary batteries are disposable, and once they are discharged, they are discarded. Alkaline manganese batteries, commonly used in remote controls and watches, are typical examples. Secondary batteries can be recharged and reused after discharge. Thus, they are also referred to as rechargeable batteries. Lithium-ion batteries represent this type and are widely used as the energy source for electric vehicles and other electrical devices such as cell phones, laptops, etc. Fuel cells generate electrical energy by electrochemically reacting a fuel with an oxidizer. Unlike typical chemical batteries, fuel cells require a continuous supply of fuel and oxygen to sustain the chemical reaction that provides electricity.
A bio-battery is a type of cell that generates power through biochemical reactions involving enzymes, microorganisms, etc. A physical battery converts forms of energy such as light, heat, or nuclear power into electricity. Examples of physical batteries include solar cells, which directly generate electricity from sunlight, and atomic cells, which convert radiation energy into electrical energy.
| The Evolution and History of Batteries: Past, Present, and Future
⚡ The origin of the word “volt” (V)
One essential thing we check before traveling abroad is the “voltage” of the destination country, measured in “volts (V).” But do you know where this term comes from?
In 1800, the Italian physicist Alessandro Volta (1745-1827) invented the Voltaic Pile, the world’s first modern battery and a fundamental chemical battery. He created electricity by stacking alternating discs of copper and zinc interspersed with pieces of cloth soaked in a dilute sulfuric acid solution. This setup produced a steady current through the electrochemical reaction between the two different metals and the electrolyte solution. This groundbreaking invention allowed humanity to move beyond static electricity to harness flowing electric current. In recognition of his contributions, the unit of electric potential was named the “volt” after him.
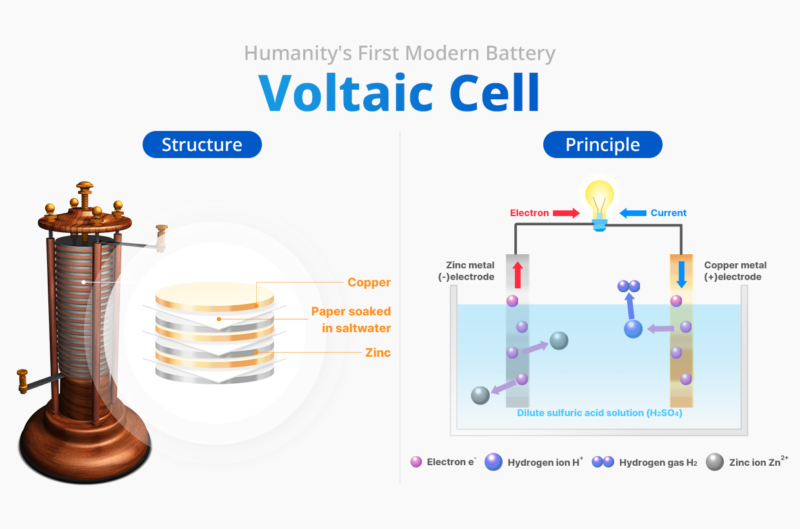
⚡ Electromagnetic Induction Experiment: Discovering Current
British physicist and chemist Michael Faraday (1791-1867) wondered if moving a magnet could generate an electric field. In 1831, he placed two coils side by side and conducted an experiment by running current through one coil, through which he discovered the phenomenon of electromagnetic induction.
Faraday’s electromagnetic rotating device fills mercury in both cups, allowing current to flow. Underneath the left cup is a magnet with a conductor fixed onto it, while the right cup had the conductor suspended above and the magnet secured in the cup. Connecting the mercury in both cups to the poles of a battery caused the magnet on the left and the conductor on the right to rotate. This electromagnetic induction experiment demonstrated that magnetic fields could generate currents, playing a crucial role in the development of our electric civilization.
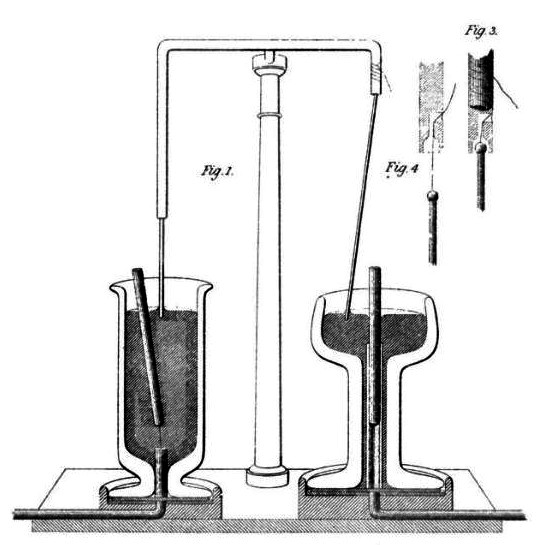
Faraday’s Electromagnetic Rotating Device (Source: Wikipedia )
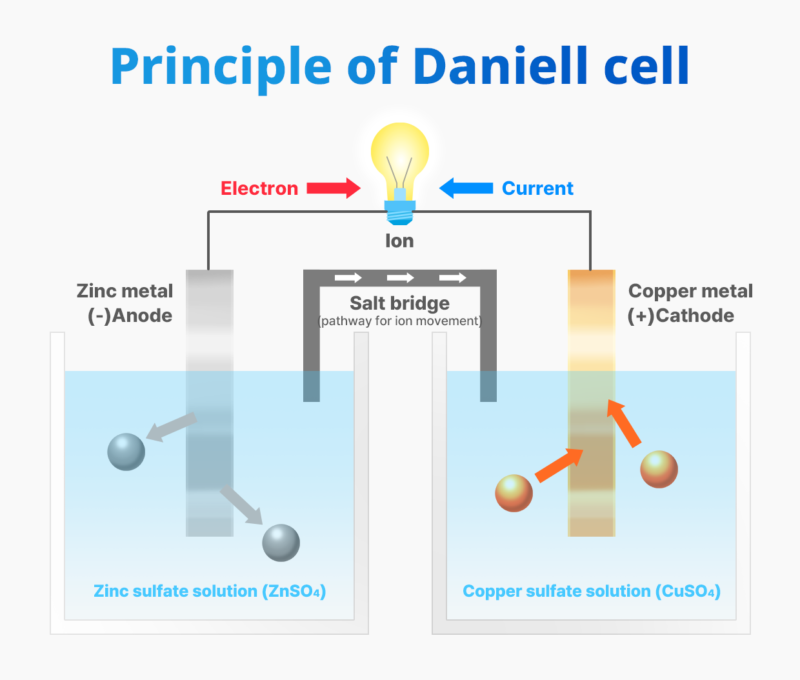
⚡ Daniell Cell, Surpassing the Voltaic Cell
In 1836, British chemist John Frederic Daniell invented a cell, which was later named “Daniell cell” after him, to solve the problem of hydrogen gas accumulation and voltage drop due to polarization in the Voltaic cell. The Daniell cell consisted of a zinc electrode in a zinc sulfate solution and a copper electrode in a copper sulfate solution, connected by a salt bridge*. This design provided a stable supply of current for the communication devices of the time.
(*) Salt Bridge: A pathway that allows free movement of cations and anions. The salt bridge contains potassium chloride (KCl) or potassium nitrate (KNO₃). When there is an increase in cations at the anode (negative electrode), the nitrate ions from the salt bridge move to balance the ions. When there is an increase in anions at the cathode (positive electrode), the potassium ions from the salt bridge move to maintain ion equilibrium.
Thus, the secondary batteries used today were reached through numerous processes. It was the result of continuous improvement and innovation based on past technologies.
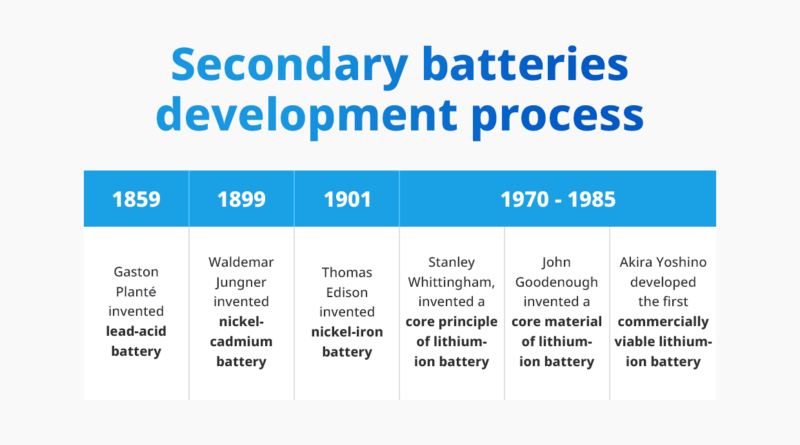
The world’s first secondary battery emerged 59 years after the invention of the Voltaic cell. In 1859, French physicist Gaston Planté (1834-1889) invented the first secondary battery, the lead-acid battery. Subsequent developments in secondary batteries led to two main types: nickel-based and lithium-based, with lithium-based batteries being predominant today. Lithium-ion batteries are favored over other secondary batteries due to being lighter and smaller yet have a higher energy density.
This has been the history and development process of batteries. In the following story, we will explore more about the revolutionary inventions that brought about the mobile revolution and the era of electric vehicles: the secondary batteries. In the next part, we will delve deeper into batteries, from the four key components that make up a secondary battery to its classification and applications.










 Youtube
Youtube Facebook
Facebook Instagram
Instagram Linkedin
Linkedin