SK Innovation
SK Innovation■ SK Innovation’s Institute of Environmental Science and Technology gained a foothold for the commercialization of CO2 conversion to CO by the independent development of an electrocatalyst.
– Published in a top-class academic journal in environmental studies as a spotlighted method to reduce greenhouse gases
■ “In combination with the R&D potential in energy & chemical study steadily accumulated, the company is going to develop, further, and apply carbon reduction technologies”
SK Innovation’s Institute of Environmental Science and Technology independently developed an electrocatalyst technology for CO2 (carbon dioxide) conversion to CO (carbon monoxide), gaining a foothold for commercialization. The finding was published on the website of Applied Catalysis B: Environmental on September 18.
Electrochemical conversion uses electrolysis to convert one of the typical greenhouse gases, CO2, into value-added carbon compounds, such as CO. This technology is spotlighted as a promising way to reduce greenhouse gases. CO is used as a source material to produce various chemical products, such as acetic acid, plastics. It is also used to produce alternative fuels, such as synthetic fuel oil, methanol.
A lot of companies in the industry are already conducting research projects to replace highly active but very expensive metallic electrocatalysts, such as gold or silver with less expensive ones, such as iron, nickel, and improve their performance by managing them as precisely as inatomic level.
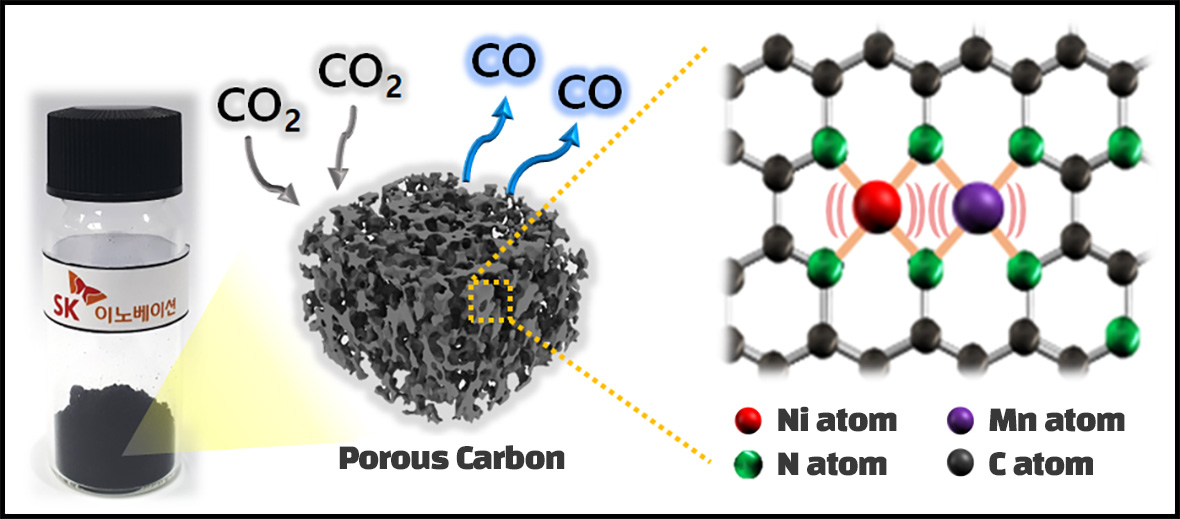
While the existing catalysts are like chunks of hundreds of bonded atoms, recently developed single-atom catalysts (SACs) are made by isolating the atoms one by one, making the reaction drastically efficient. SK Innovation’s Institute of Environmental Science and Technology has made an advancement even further than that. They developed diatomic electrocatalysts by bonding two adjacent metal atoms into a pair, resulting in further enhancement in the performance.
Based on their observation that the catalyst made with one nickel atom and one manganese atom attached to each other creates a synergy in the catalytic reaction, the research team successfully developed a new technology that not only reduces the energy used to resolve CO2 but also converts more than 98% of the CO2 into CO. And this is one of the highest conversion rates among the published results so far.
Besides securing capabilities to develop next-generation catalysts, SK Innovation plans to expand the scale of electrochemical reactors, acquire technologies that can be mass-produced, and, eventually, make a contribution to carbon neutrality through cooperation with external professional institutions.
Lee Seong-jun, Head of SK Innovation’s Institute of Environmental Science and Technology, said, “The finding resulted from the application of SK Innovation’s technological prowess in catalysts, accumulated from energy and chemical R&D for decades, to the development of carbon reduction technologies.” He added, “SK Innovation will continuously enhance its core R&D capabilities in various fields such as catalyst development, production processes, and synthesis, and apply findings to the development of carbon neutralization technologies.”
[Photo]
(Photo 1) The cover image of the study on the online journal

(Photo 2) A mimetic diagram that shows Co2 being resolved to CO in a diotomic catalyst that consists of neighboring nikel/magnese metal atom










 Youtube
Youtube Facebook
Facebook Instagram
Instagram Linkedin
Linkedin